Survivor Views of Pharmacogenomic Testing
Overview
The American Cancer Society Cancer Action Network (ACS CAN) empowers advocates across the country to make their voices heard and influence evidence-based public policy change, as well as legislative and regulatory solutions that will reduce the cancer burden. As part of this effort, ACS CAN deploys surveys to better understand cancer patient and survivor experiences and perspectives, through our Survivor Views research panel. The panel is a group of cancer patients and survivors who respond to regular surveys and provide important insights to support ACS CAN’s advocacy work at all levels of government.
Fielded June 26-July 19, 2023, our latest survey explores awareness of and attitudes toward pharmacogenomic (PGx) testing, a form of genetic testing to better understand a patient’s risk for drug reactions. The web-based survey was conducted among 1,155n cancer patients and survivors nationwide who have been diagnosed with or treated for cancer in the last seven years. The full text of these questions and more methodological detail follow the summary of the results below.
Key Findings
- 42% of cancer patients and survivors were aware of PGx testing before this survey
- Most of those (45%) learned about PGx testing from a provider, while just 4% of those aware of PGx testing learned about it in the information about drugs they took
- Once informed, 70% said they would be concerned about being administered a drug with a low risk of severe side effects without first being given PGx testing.
Detailed Survey Findings
The questionnaire included the following definition of PGx:
Each person has a slightly different inherited genetic makeup that can cause different people to have different responses to the same drug. These differences may impact the effectiveness of a drug or the side effects a person may experience. (Note: this is different from biomarker testing of a tumor to help determine which drug may be used)
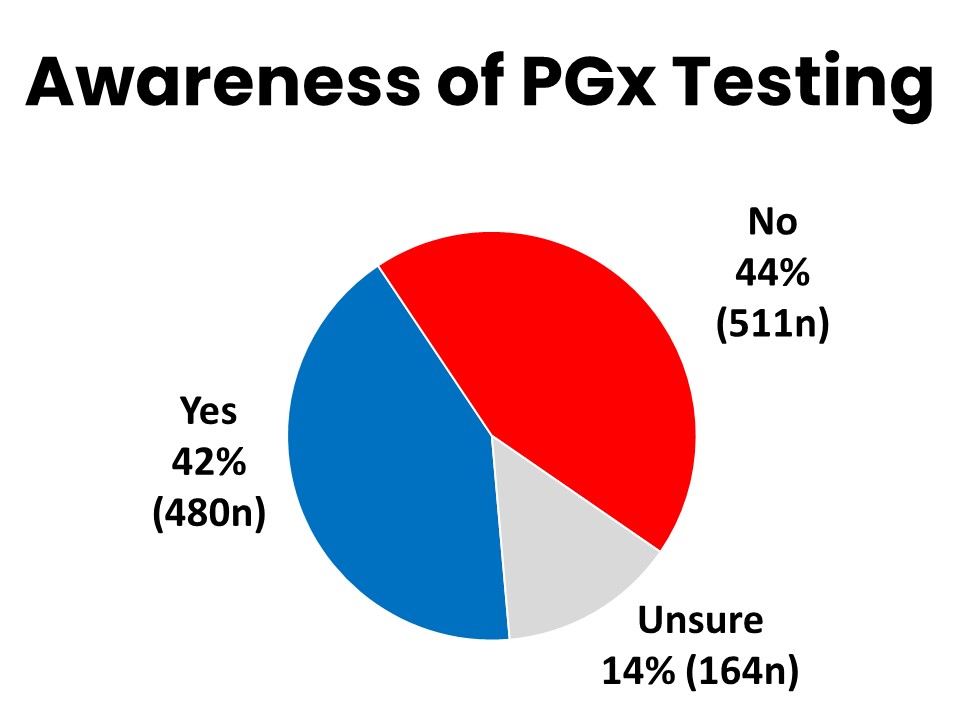
Four-in-Ten Aware of PGx Testing
Based on the definition above, 42% of cancer patients and survivors surveyed (480n) said they were aware before this survey that it is possible to conduct this testing to understand potential differences in drug response due to genetic factors. Forty-four percent (511n) said they were not previously aware of this. Those most likely to be familiar with PGx testing tended to be highly educated (52% of those with a post graduate degree report having heard of PGx testing), and have higher household income (50% of those with annual household income over $125,000).
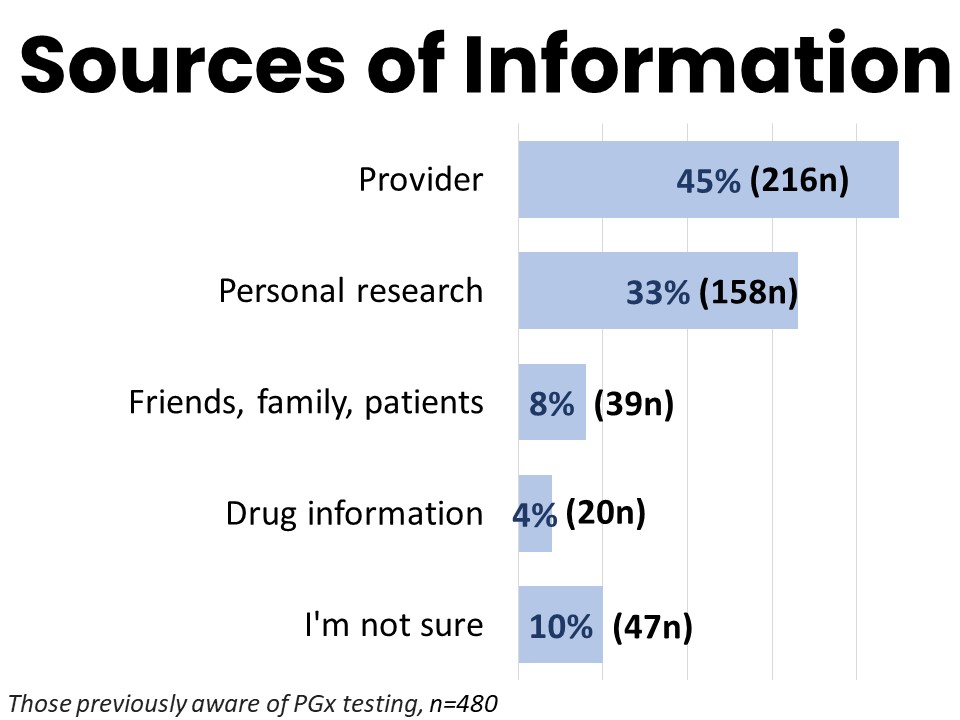
Most Learn About PGx from Their Provider
Among those previously aware of PGx testing, 45% (216n) report hearing about it from a provider (including doctors, nurses, and pharmacists). One third (158n) reported learning about PGx testing through their own personal research, such as online, in books, articles, or in school. Eight percent (39n) discovered PGx testing through friends or family while 4% (20n) read about it in the information about drugs they took.
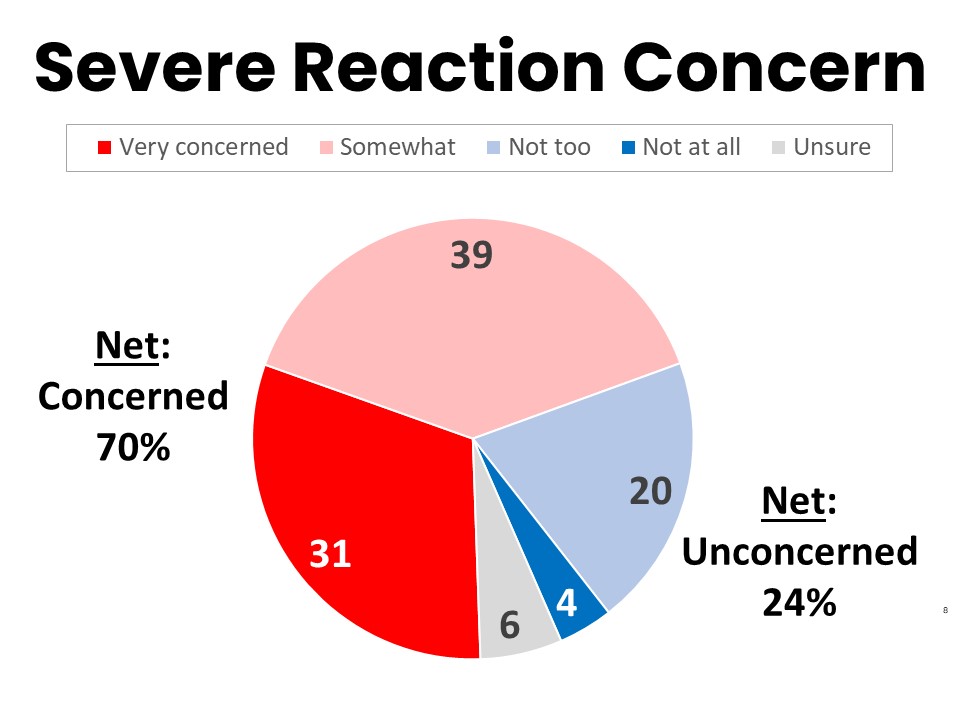
Once Aware, 70% Would be Concerned About Being Prescribed a Drug Associated with Severe Reactions Without PGx Testing
After the awareness question, respondents were asked how concerned they would be about a provider administering a drug that a small number of patients (1%) have a severe reaction to without first testing the respondent’s potential for a severe reaction. A combined 70% (805n) said they would be concerned, with 31% (360n) very concerned and 39% (445n) somewhat concerned about taking the drug without PGx testing. Fewer than one quarter reported not being concerned after learning that PGx testing might be able to identify their risk of a severe reaction.
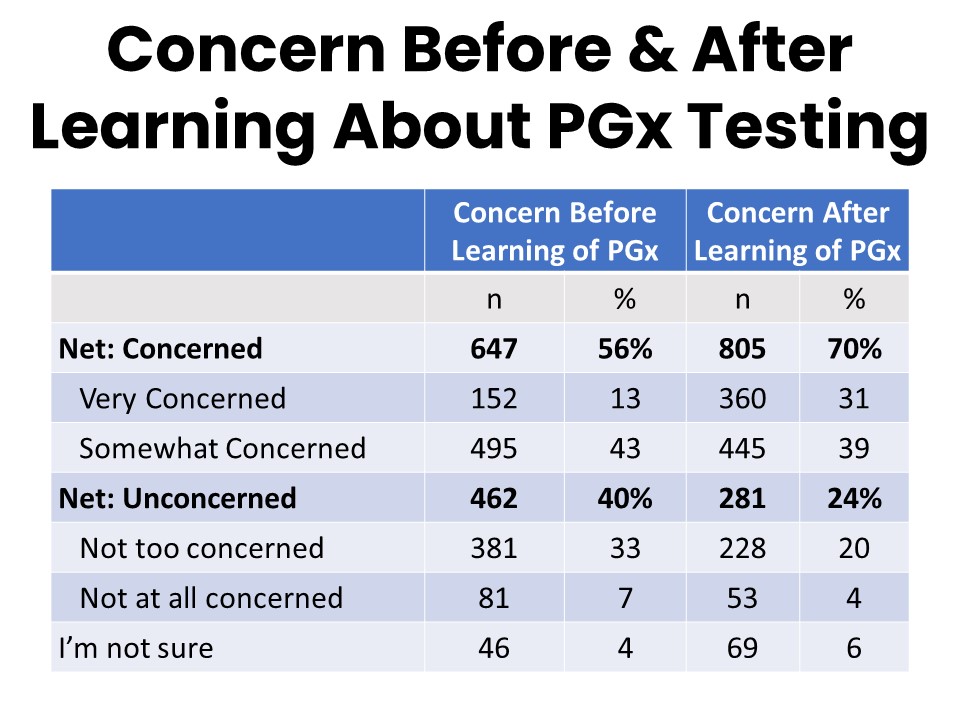
Prior to the questions about PGx, 56% of respondents reported that they would be very or somewhat concerned about taking a drug that a small number of patients (1%) have a severe reaction to. Forty percent said they would be not very or not at all concerned. The table below presents the responses to that question regarding concern about taking the drug before PGx was mentioned in the survey, as well as the questions administered after PGx was mentioned regarding concern about being prescribed the drug without being tested for a potential severe reaction.
Methodology
ACS CAN’s Survivor Views research initiative was designed to support the organization’s efforts to end suffering and death from cancer through public policy advocacy. Data provided by cancer patients and survivors as part of this project allows for a greater understanding of their experiences and opinions on cancer-related issues and gives voice to cancer patients and survivors in the shaping and advocating of public policies that help prevent, detect, and treat cancer and promote a more positive quality of life for those impacted.
To ensure the protection of all participants in this initiative all research protocols, questionnaires, and communications are reviewed by the Morehouse School of Medicine Institutional Review Board.
The survey population is comprised of individuals who meet the following criteria:
- Diagnosed with and/or treated for cancer within the last seven years
- Over the age of 18 (parents of childhood cancer survivors were invited to participate on behalf of their minor children)
- Reside in the US or US territories
Potential Survivor Views participants were invited to participate through email invitations, social media promotion, and partner group outreach. Those who agreed to participate after reviewing the informed consent information completed a brief survey including demographic and cancer history information to inform analysis as well as topical questions as discussed in this document. The data were collected between June 26-July 19, 2023. A total of 1,155 cohort participants responded to the survey. Differences reported between groups are tested for statistical significance at a 95% confidence interval.
About ACS CAN
The American Cancer Society Cancer Action Network (ACS CAN) advocates for evidence-based public policies to reduce the cancer burden for everyone. We engage our volunteers across the country to make their voices heard by policymakers at every level of government. We believe everyone should have a fair and just opportunity to prevent, detect, treat, and survive cancer. Since 2001, as the American Cancer Society’s nonprofit, nonpartisan advocacy affiliate, ACS CAN has successfully advocated for billions of dollars in cancer research funding, expanded access to quality affordable health care, and advanced proven tobacco control measures. We stand with our volunteers, working to make cancer a top priority for policymakers in cities, states and our nation’s capital. Join the fight by visiting www.fightcancer.org.