Survivor Views on Artificial Intelligence and FDA Medical Product Reviews
Overview
The American Cancer Society Cancer Action Network (ACS CAN) empowers advocates across the country to make their voices heard and influence evidence-based public policy change, as well as legislative and regulatory solutions that will reduce the cancer burden. As part of this effort, ACS CAN deploys surveys to better understand cancer patient and survivor experiences and perspectives, through our Survivor Views research panel. The panel is a group of cancer patients and survivors who respond to regular surveys and provide important insights to support ACS CAN’s advocacy work at all levels of government.
Fielded June-July, 2025, our latest survey explores cancer patients’ and survivors’ perspectives related to the review and approval of new medical drugs and devices, as well as opinions of AI and it’s use in healthcare. The web-based survey was conducted among 1,267n cancer patients and survivors nationwide who have been diagnosed with or treated for cancer in the last seven years. More methodological detail follows at the end of this report.
Key Findings
- Eighty percent of cancer patients and survivors agree that the industries making FDA-regulated products should provide some of the cost of FDA’s public health oversight
- Cancer patients and survivors mirror the American public in their hesitancy to embrace AI, with 51% reporting they are more concerned than excited about the technology compared to only 5% who are more excited than concerned.
- Respondents have mixed expectations of the impact of AI on health care, with a plurality (39%) predicting the impact over the next 20 years to be equally positive and negative.
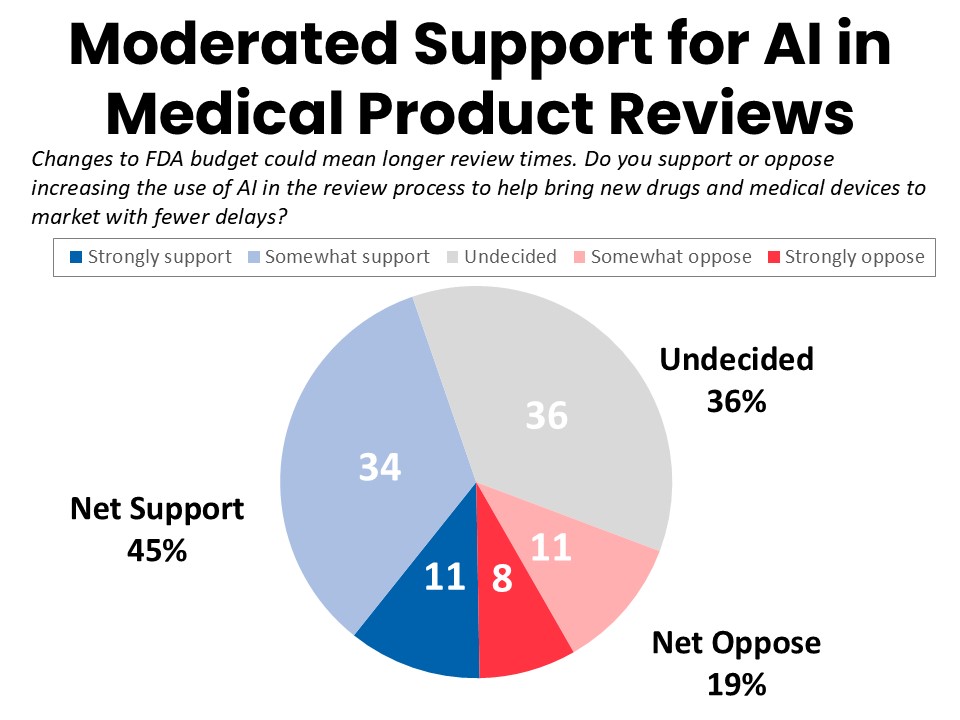
- Cancer patients and survivors offer tempered support for the use of AI in FDA product reviews if funding cuts significantly extend review times, with a plurality of 36% undecided on whether they support or oppose it.
Detailed Survey Findings
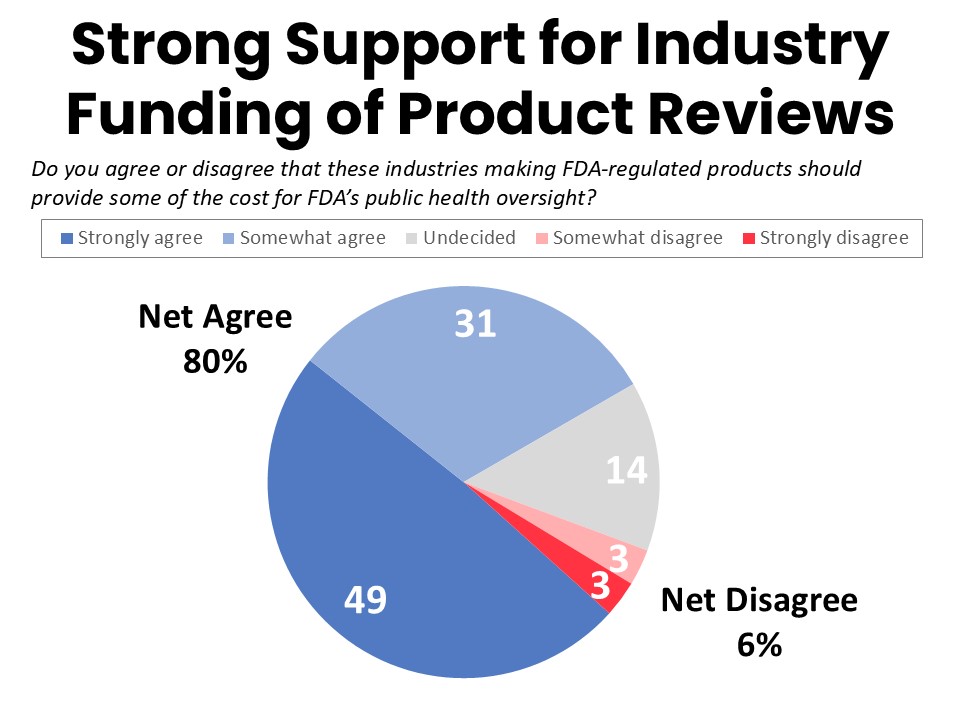
Cancer Patients and Survivors Overwhelmingly Agree Industry Should Fund Product Reviews
Eighty percent of surveyed cancer patients and survivors agree that the industries making FDA-regulated drugs and medical devices should provide some of the cost for FDA’s public health oversight. Fourteen percent are undecided while just 6% disagree.
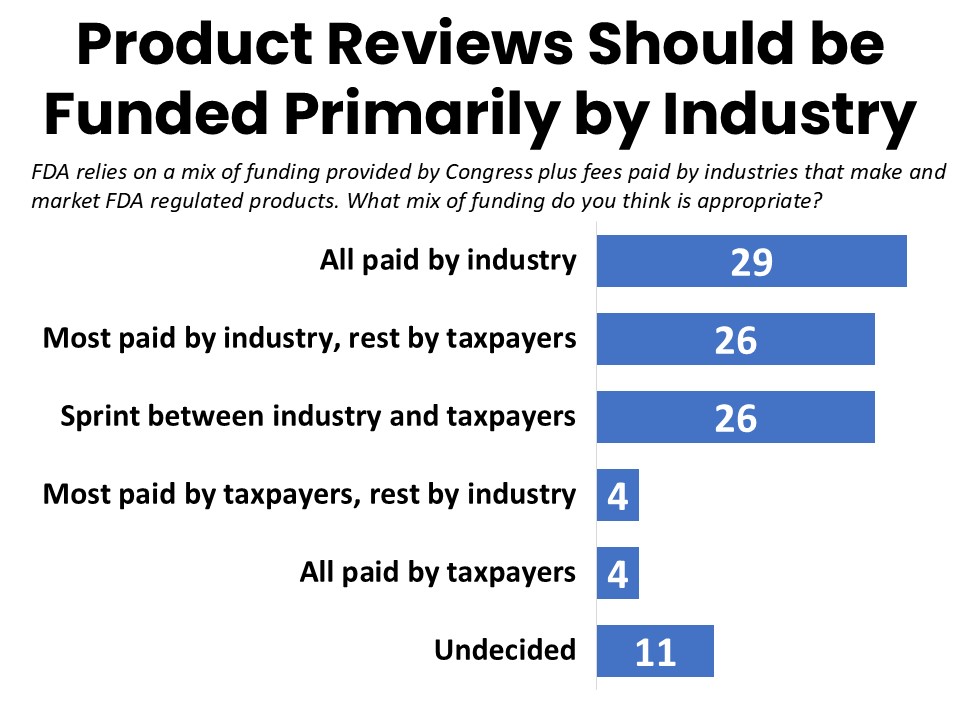
When told that FDA relies on a mix of funding provided by Congress plus fees paid by industries that make and market FDA regulated products, 55% say all or most of the funding should be provided by industry, with 29% saying FDA’s medical product reviews should be funded entirely by industry. Just over a quarter (26%) say this funding should be equally shared between industry and taxpayers, while only 8% say all or most of FDA’s medical product review budget should be provided by taxpayers. If current levels of industry funding were cut or eliminated from the budget for medical product reviews, the plurality (45%) say they are unsure what should happen, while others are nearly evenly split between the taxpayer portion increasing moderately with some delays in review times (20%), significantly enough to avoid delays in review (18%), or not at all with significant impact on review times (17%).
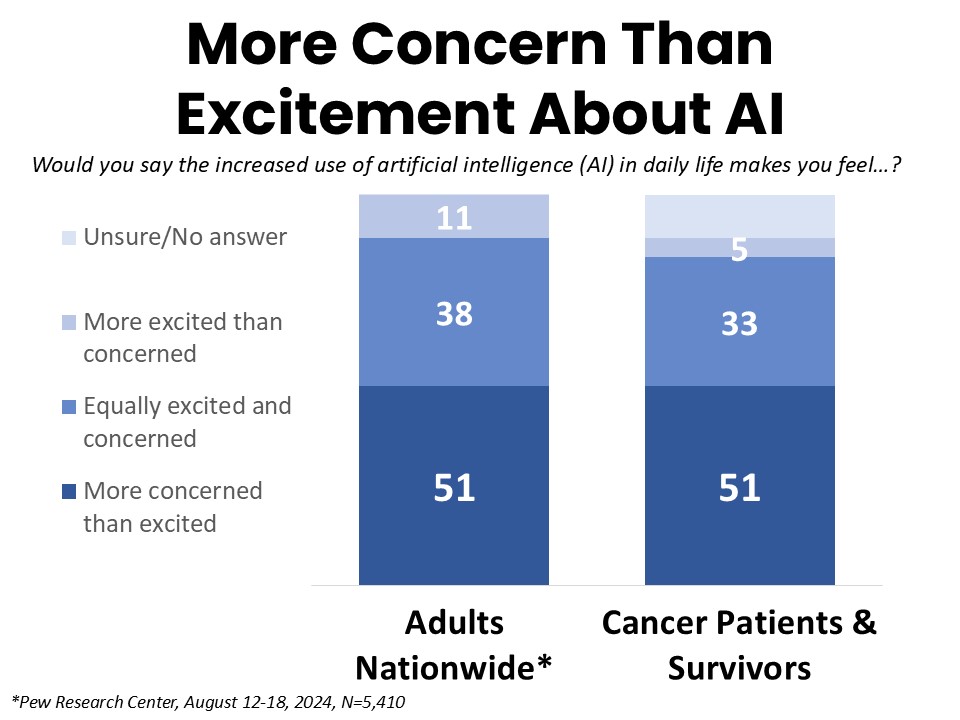
Cancer Patients and Survivors Mirror the American Public in Their Hesitancy to Embrace AI
Mirroring the sentiment of the US national adult population as reported by Pew Research Center, the majority of cancer patients and survivors surveyed are more concerned than excited about the use of AI in daily life (51%), compared to just 5% who report feeling more excited than concerned. One-third are equally excited and concerned. Respondents under age 45 are the least concerned, with 50% of this group reporting equal concern and excitement, while Black survey respondents report the highest levels of concern (58% more concerned than excited). Similar to the above referenced Pew study, 36% of respondents report having heard or read “a lot” about AI, and that segment is also more concerned than excited (46%, compared to 9% more excited than concerned).
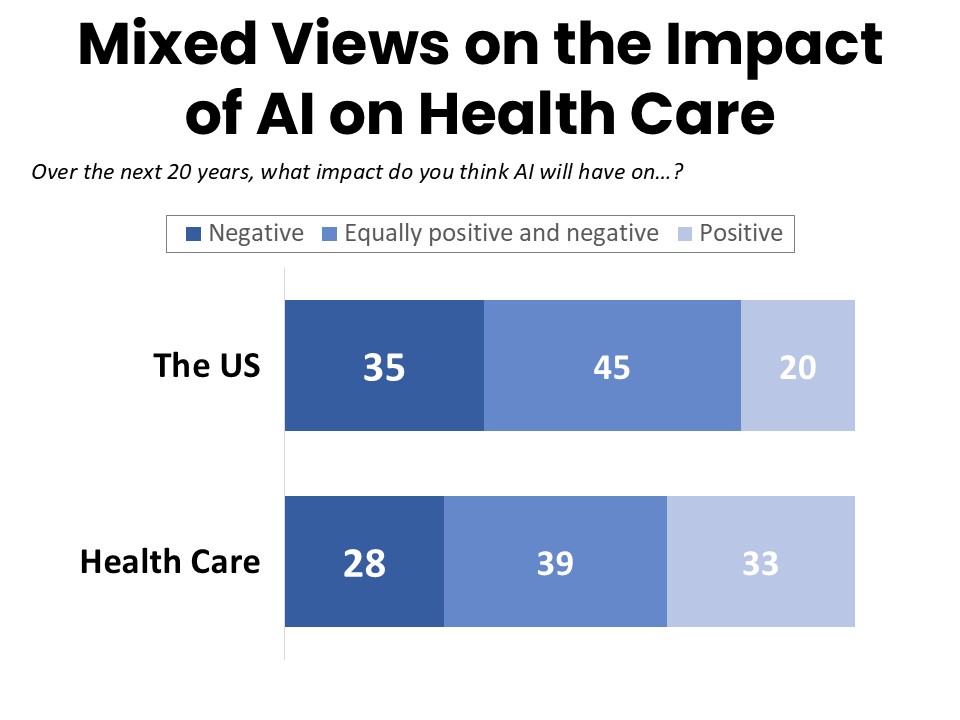
Respondents Have Mixed Expectations for the Impact of AI
A plurality of cancer patients and survivors expect the general impact of AI on the US over the next 20 years to be mixed, with 45% predicting it will be equally positive and negative. Thirty-five percent believe the impact will be more negative and 20% expect a more positive impact. While survey respondents are somewhat more optimistic about the impact of AI on health care, the plurality are again mixed at 39%, with 33% anticipating a positive impact of AI on healthcare and 28% expecting a more negative impact.
Cancer Patients and Survivors Offer Cautious Support for the Use of AI in Medical Product Reviews
Support for the use of AI in medical product reviews reflects the hesitancy and mixed expectations about the technology, although those impacted by cancer are understandably eager to have access to the latest treatments and therapies with as little delay as possible. If changes to the FDA budget result in longer review times for new drugs and medical devices, survey respondents express tempered support for the use of AI in the review process to bring new drugs and medical devices to market with fewer delays. A 36% plurality say they are undecided on this use of AI, while nearly as many (34%) somewhat support the idea and 11% somewhat oppose. Another 11% strongly support increasing the use of AI in FDA’s review process, while 8% strongly oppose it.
Methodology
ACS CAN’s Survivor Views research initiative was designed to support the organization’s efforts to end suffering and death from cancer through public policy advocacy. Data provided by cancer patients and survivors as part of this project allows for a greater understanding of their experiences and opinions on cancer-related issues and gives voice to cancer patients and survivors in the shaping and advocating of public policies that help prevent, detect, and treat cancer and promote a more positive quality of life for those impacted.
To ensure the protection of all participants in this initiative all research protocols are reviewed by the Morehouse School of Medicine Institutional Review Board.
The survey population is comprised of individuals who meet the following criteria:
- Diagnosed with and/or treated for cancer within the last seven years
- Over the age of 18 (parents of childhood cancer survivors were invited to participate on behalf of their minor children)
- Reside in the US or US territories
Survivor Views participants are invited to participate through email, direct mail, social media, and outreach to communities and partners engaged with cancer patients and survivors. Those who agree to participate after reviewing the informed consent information are invited to join the Survivor View’s research cohort and participate in future surveys. This survey also includes cancer patients and survivors nationwide from probability and non-probability panels. The data for this survey were collected between June 4-July 28, 2025. A total of 1,267 participants responded to the survey.
About ACS CAN
The American Cancer Society Cancer Action Network (ACS CAN) advocates for evidence-based public policies to reduce the cancer burden for everyone. We engage our volunteers across the country to make their voices heard by policymakers at every level of government. We believe everyone should have a fair and just opportunity to prevent, detect, treat, and survive cancer. Since 2001, as the American Cancer Society’s nonprofit, nonpartisan advocacy affiliate, ACS CAN has successfully advocated for billions of dollars in cancer research funding, expanded access to quality affordable health care, and advanced proven tobacco control measures. We stand with our volunteers, working to make cancer a top priority for policymakers in cities, states and our nation’s capital. Join the fight by visiting www.fightcancer.org.