Survivor Views on the Composition of Clinical Trials
Overview
The American Cancer Society Cancer Action Network (ACS CAN) empowers advocates across the country to make their voices heard and influence evidence-based public policy change, as well as legislative and regulatory solutions that will reduce the cancer burden. As part of this effort, ACS CAN deploys surveys to better understand cancer patient and survivor experiences and perspectives, through our Survivor Views research panel. The panel is a group of cancer patients and survivors who respond to regular surveys and provide important insights to support ACS CAN’s advocacy work at all levels of government.
Fielded June 26-July 19, 2023, our latest survey explores the impact of cancer diagnosis and treatment on the social connections of cancer patients and survivors. In addition, this survey also assessed awareness of and attitudes toward pharmacogenomics, or genetic testing to better understand a patient’s risk for drug reactions. The web-based survey was conducted among 1,155n cancer patients and survivors nationwide.
Key Findings
Representation is important to cancer patients and survivors when it comes to the composition of clinical trials. Four-in-five (79%) say it is important that a drug they are prescribed has been tested in people whose health is similar to theirs, with 57% saying this is very important and 22% somewhat important. Similarly, 76% agree that it is important the drug has been tested in people in their age group (50% very important). Gender representation ranks next in terms of importance, with 73% saying it is important a drug they have been prescribed has been tested in people of their gender (52% very important). Women (54%) are more likely than men (31%) to say it is very important that a drug is tested in patients who share their gender.
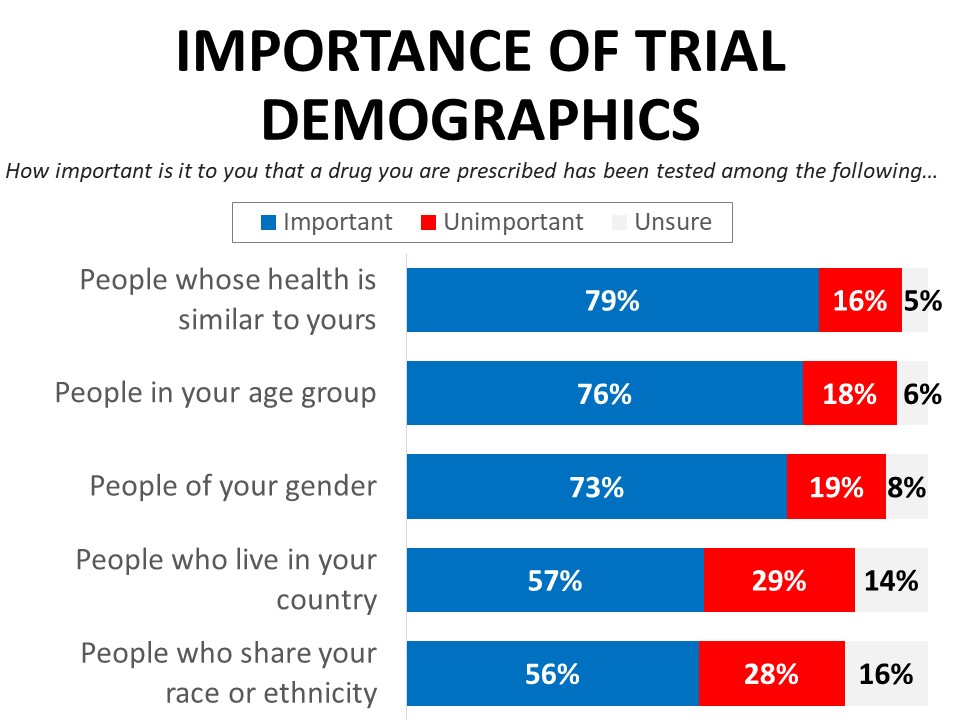
Nationality, race, and ethnicity are viewed as somewhat less important than health condition, age, and gender when it comes to trial diversity, though still important. Fifty-seven percent say it is important the drug has been tested in people who live in their country (35% very important) and 56% agree it is important the drug has been tested in people who share their racial or ethnic identity (33% very important), with about half as many saying these demographics are not important. In general, people of color are much more likely to rate all of the categories of trial diversity we tested as very important compared to white respondents.
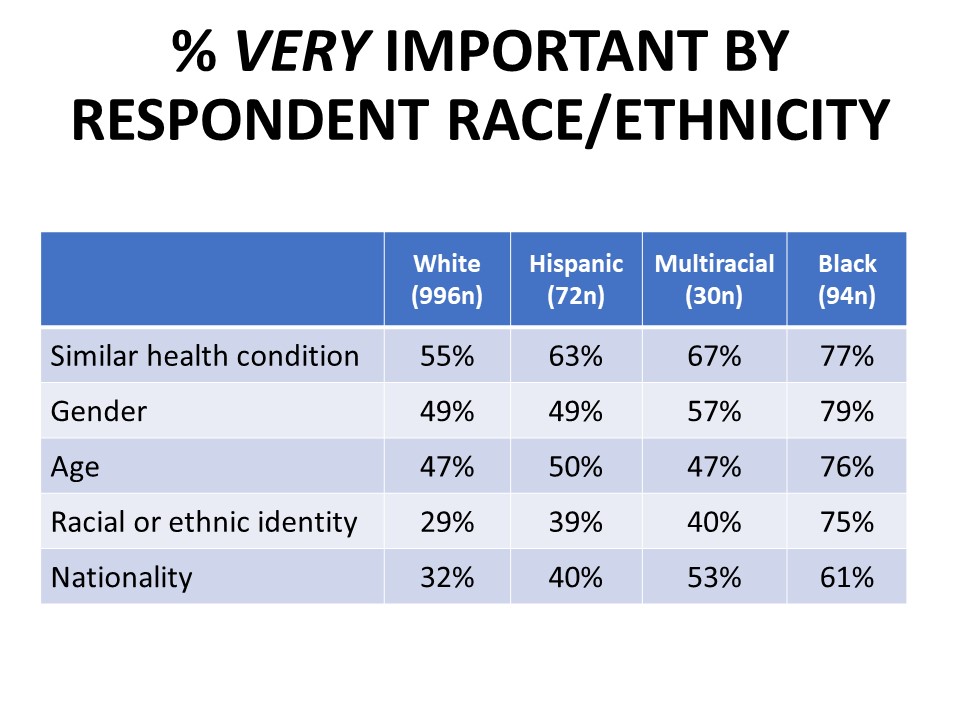
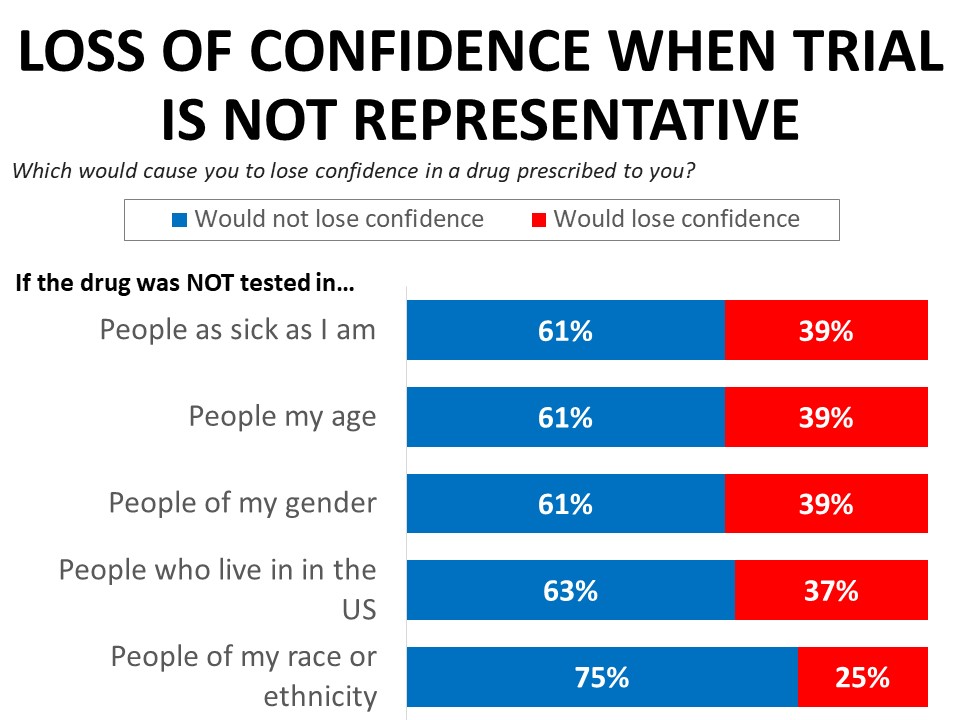
Learning that a drug was not tested in those of comparable health or people the same age or gender would erode confidence in a drug the respondent was prescribed, with 39% saying they would lose confidence in the drug. Thirty-seven percent say they would lose confidence in the drug if it was not tested in people who live in the US, and 25% say they would lose confidence if the drug was not tested in people of their race or ethnicity. The erosion in confidence when trials don’t reflect the patient’s race is much more significant among black cancer patients and survivors, among whom 61% would lose confidence in a drug not tested in patients who share their race or ethnicity, compared to just 21% of white respondents.
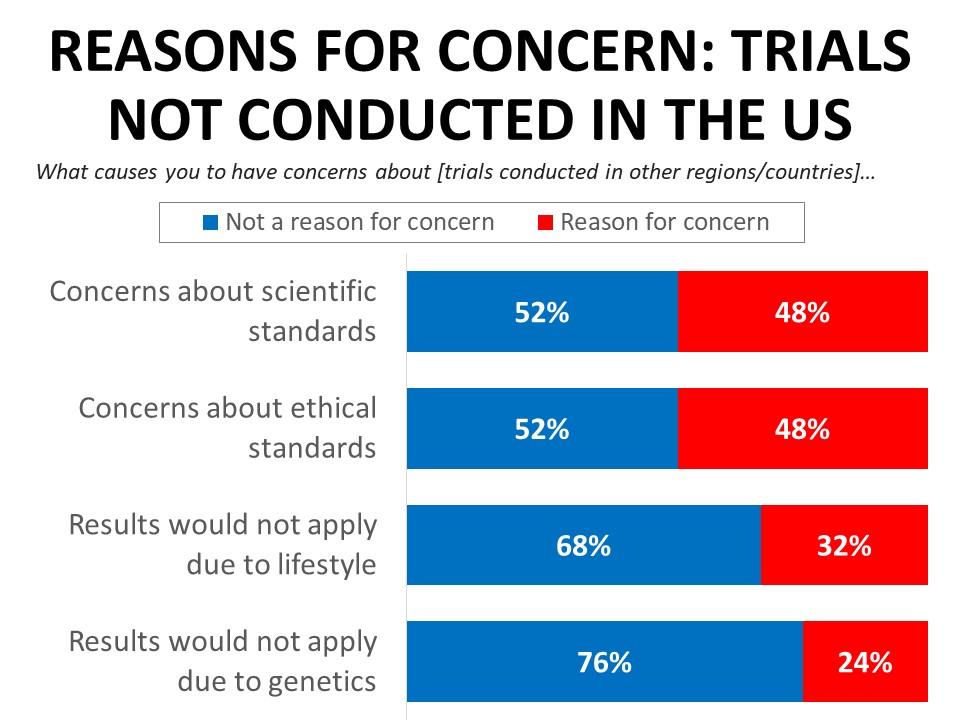
While the majority of respondents (63%) would not lose confidence in a drug that was not tested among patients in the US, 48% of those with concerns about drugs tested only in other countries cite concerns about scientific or ethical standards. Just under one-third (32%) say they would be concerned about whether the results apply to them due to differences in lifestyles, diets, and other health conditions, and just under one-quarter (24%) would have concerns about whether the results would apply due to differences in race, ethnicity, or genetics.
Methodology
ACS CAN’s Survivor Views research initiative was designed to support the organization’s efforts to end suffering and death from cancer through public policy advocacy. Data provided by cancer patients and survivors as part of this project allows for a greater understanding of their experiences and opinions on cancer-related issues and gives voice to cancer patients and survivors in the shaping and advocating of public policies that help prevent, detect, and treat cancer and promote a more positive quality of life for those impacted.
To ensure the protection of all participants in this initiative all research protocols, questionnaires, and communications are reviewed by the Morehouse School of Medicine Institutional Review Board.
The survey population is comprised of individuals who meet the following criteria:
- Diagnosed with and/or treated for cancer within the last seven years
- Over the age of 18 (parents of childhood cancer survivors were invited to participate on behalf of their minor children)
- Reside in the US or US territories
Potential Survivor Views participants were invited to participate through email invitations, social media promotion, and partner group outreach. Those who agreed to participate after reviewing the informed consent information completed a brief survey including demographic and cancer history information to inform analysis as well as topical questions as discussed in this document. The data were collected between June 26-July 19, 2023. A total of 1,155 cohort participants responded to the survey. Differences reported between groups are tested for statistical significance at a 95% confidence interval.
About ACS CAN
The American Cancer Society Cancer Action Network (ACS CAN) advocates for evidence-based public policies to reduce the cancer burden for everyone. We engage our volunteers across the country to make their voices heard by policymakers at every level of government. We believe everyone should have a fair and just opportunity to prevent, detect, treat, and survive cancer. Since 2001, as the American Cancer Society’s nonprofit, nonpartisan advocacy affiliate, ACS CAN has successfully advocated for billions of dollars in cancer research funding, expanded access to quality affordable health care, and advanced proven tobacco control measures. We stand with our volunteers, working to make cancer a top priority for policymakers in cities, states and our nation’s capital. Join the fight by visiting www.fightcancer.org.